Sensors | Free Full-Text | Effect of Pd-Sensitization on Poisonous Chlorine Gas Detection Ability of TiO2: Green Synthesis and Low-Temperature Operation

Insight into the performance of UV/chlorine/TiO2 on carbamazepine degradation: The crucial role of chlorine oxide radical (ClO•) - ScienceDirect

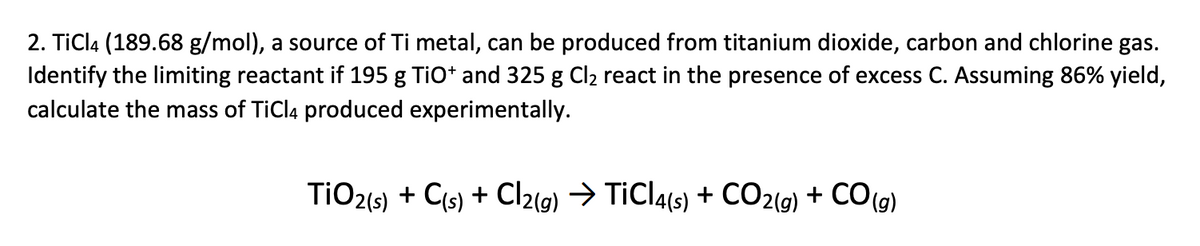
TiO2 +C+2Cl2 →TiCl4 +CO2 What mass of Cl2 is needed to react with 1.25 mol TiO2? What mass of C is needed to react with 1.252 mol TiO2? - GuideChem

High-resolution TEM images of Pt/TiO2 (a), Au/TiO2 (b), Pd/TiO2 (c) and... | Download Scientific Diagram

Review 3.pdf - 1. Titanium dioxide TiO2 reacts with carbon and chlorine to give gaseous TiCl4: TiO₂ 2 C 2 Cl sub 4 → TiCl₄ 2 CO The reaction | Course Hero

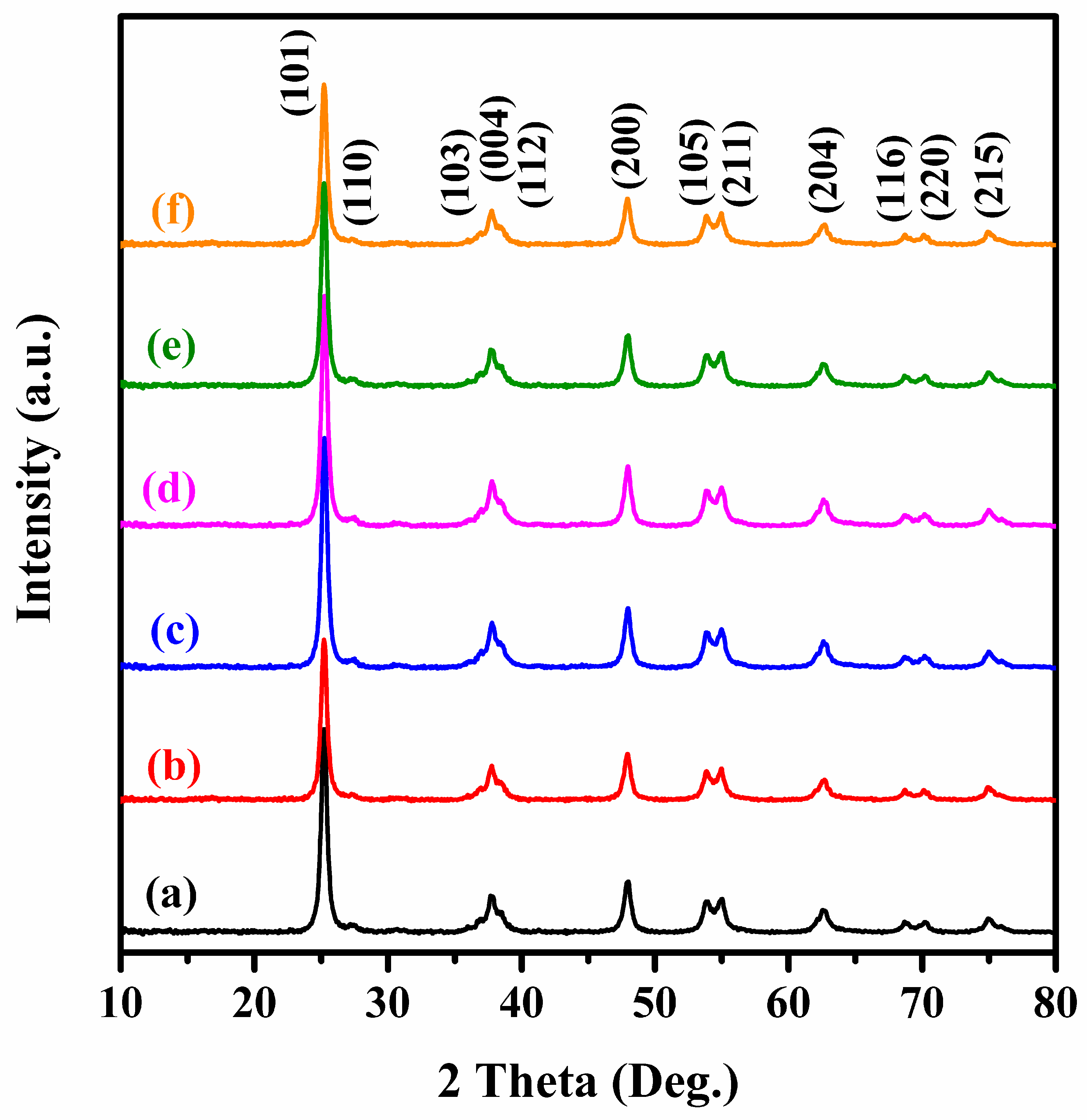
First-Principles Calculations of Adsorption Reactions of C and Cl2 on TiO2 (001) Surface with Bridge-Oxygen Defect in Fluidized Chlorination | springerprofessional.de

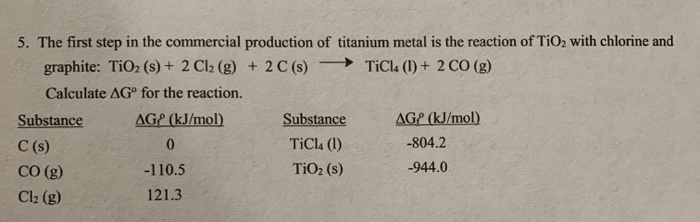
SOLVED: QUESTION Consider the following eaction: 2 Ti02 +3C+4Cl2 2 TiCl4 CO2 2 CO If 12 moles TiO2' moles C, and 10 moles Clz are reacted, the limiting reagent will be CO2

Titanium, which is used to make airplane engines and frames can be obtained from titanium tetrachloride, which in turn is obtained from titanium oxide by the following process: 3TiO2(s) + 4C(s) +
Catalysts | Free Full-Text | AuPd/3DOM TiO2 Catalysts: Good Activity and Stability for the Oxidation of Trichloroethylene

One-Pot Approach for the Synthesis of Water-Soluble Anatase TiO2 Nanoparticle Cluster with Efficient Visible Light Photocatalytic Activity | The Journal of Physical Chemistry C


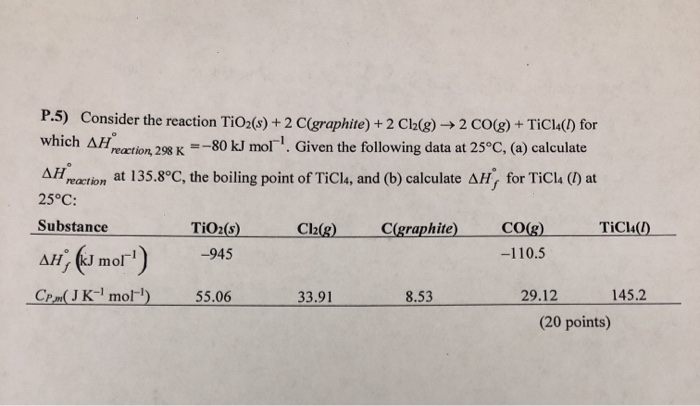




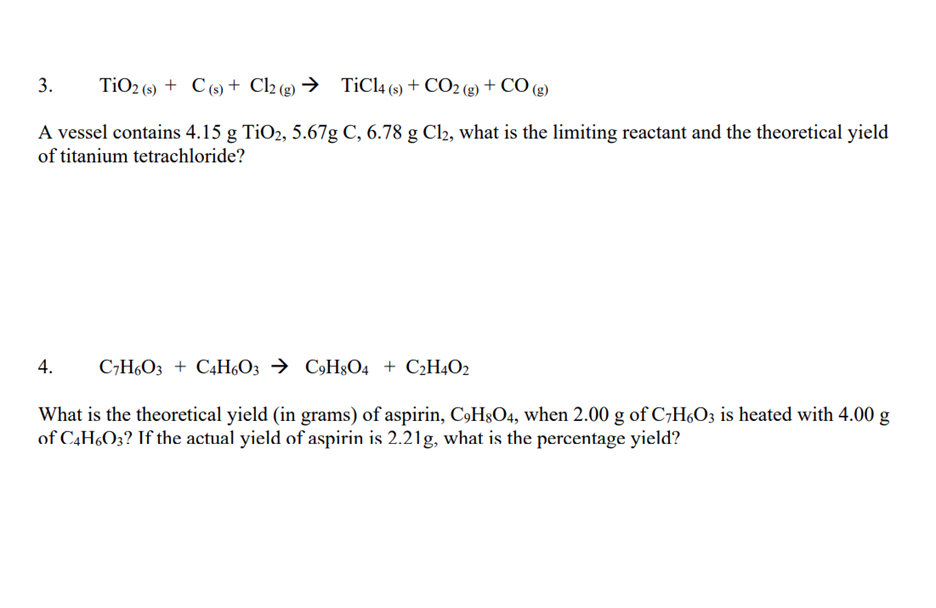
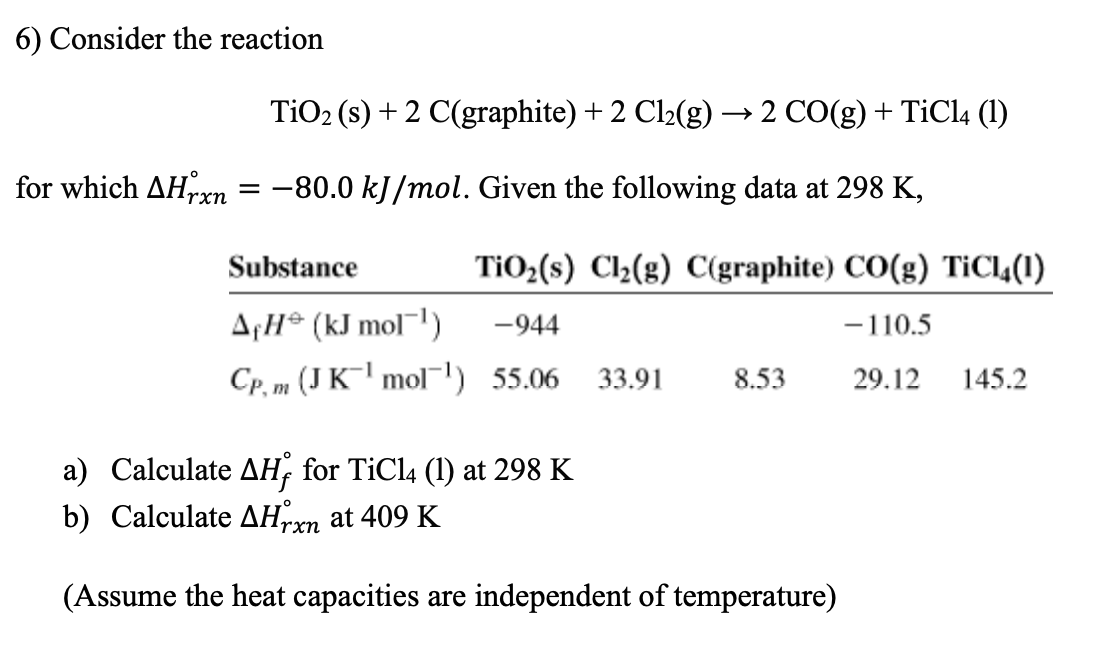

![Solved] . Homework - Week 9 QUESTION 1. Balance each equation. a. Ni(s) +... | CliffsNotes Solved] . Homework - Week 9 QUESTION 1. Balance each equation. a. Ni(s) +... | CliffsNotes](https://coursehero.s3.amazonaws.com/qattachments_aeaacc4dafff152758f37a85c243201fb41ca0c1.png?X-Amz-Content-Sha256=UNSIGNED-PAYLOAD&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIAIAYW2E6VOLDTI35A%2F20230329%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Date=20230329T191552Z&X-Amz-SignedHeaders=host&X-Amz-Expires=60&X-Amz-Signature=b57c163233b054114b34ba2b1b0ca402f4e6c43f0fda005b5a1ad867642bc4bd)