When you heat a substance, you are transferring energy into it by placing it in contact with surroundings that have a higher temperature. - ppt download

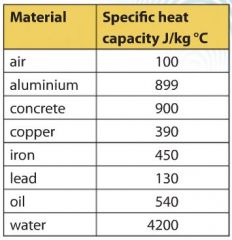
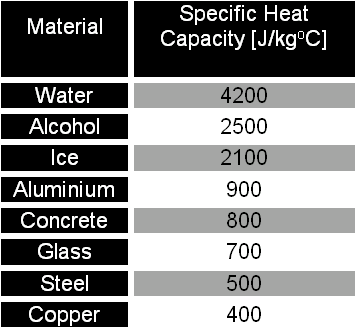
The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity

10 g of ice at 0^∘C absorbs 5460 J of heat energy to melt and change to water at 50^∘C . Calculate the specific latent heat of fusion of ice. Specific heat

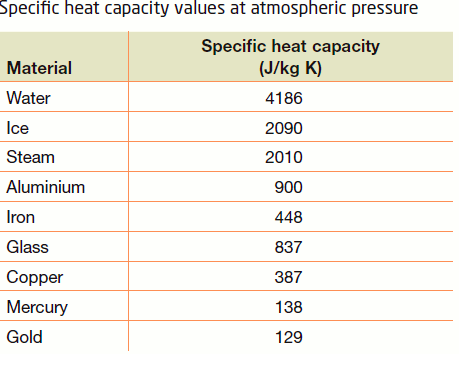
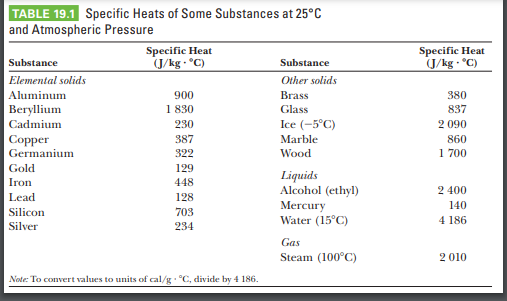
SOLVED: Specific heat capacity of water =4186J kg 1K-1 Specific heat capacity of ice =2090 J kg 1K-1 Latent heat of fusion of ice 335 kJ kg-1 Latent Heat of vaporization of