Book 4C: 2022 Good Manufacturing Practice in the European Union, Refer – Clinical Research Resources, LLC
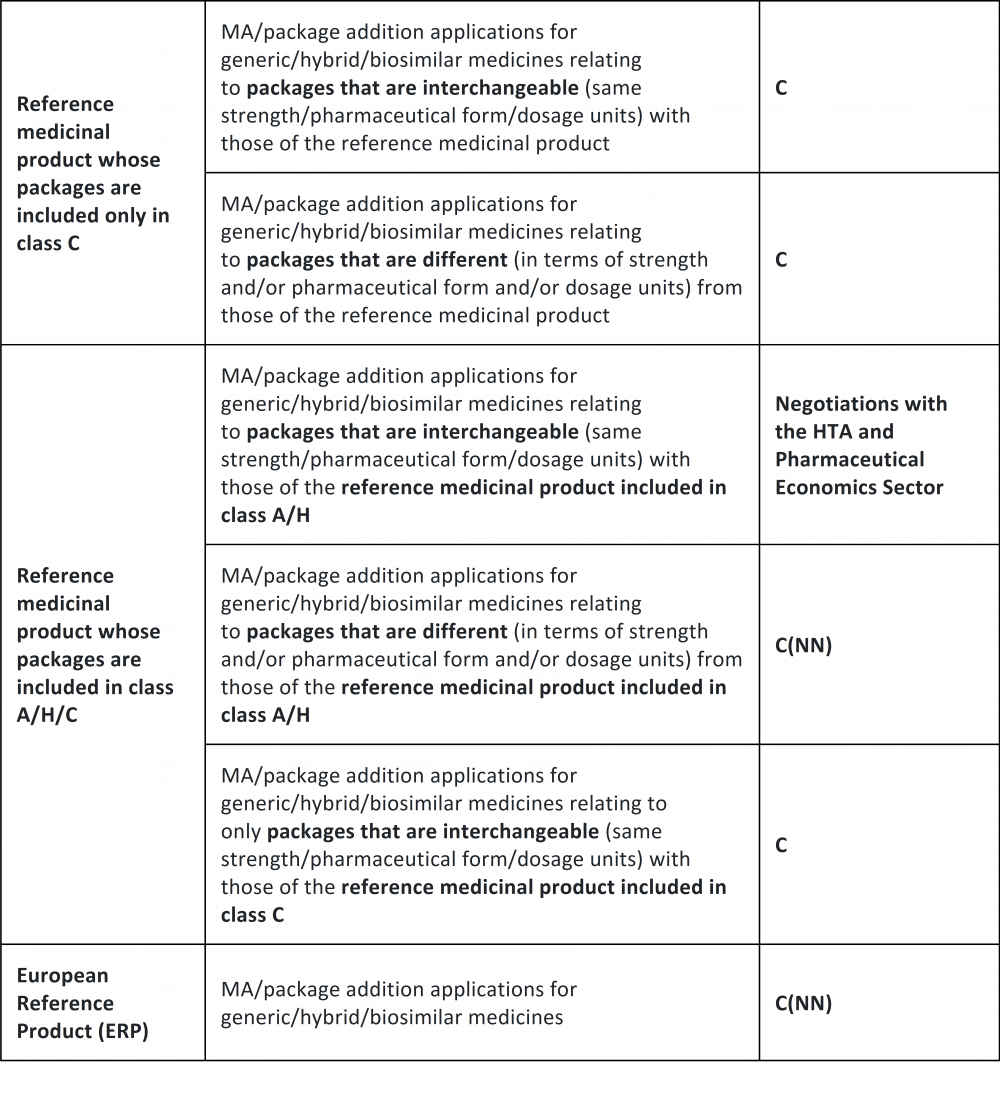
The Italian Medicines Agency provides additional information on the new simplified classification procedure for generics and biosimilars - Portolano Cavallo

Marketing authorization and licensing of medicinal products in EU: Regulatory aspects - ScienceDirect

Impact of the European Union on access to medicines in low- and middle-income countries: A scoping review - The Lancet Regional Health – Europe
CMD(h) WORKING DOCUMENT - INFORMATION TO BE SUBMITTED BY THE MEMBER STATE OF THE EUROPEAN REFERENCE MEDICINAL PRODUCT January 20
Marketing authorisations which are recommended for maintenance and marketing authorisation applications for which bioequivalence